Providing Key Diagnostic Testing Response Technology
FDA EUA Antigen POC Solution
Our FDA/EUA-approved Antigen POC Solution provides results in 10 minutes with 88.4% sensitivity and 100% specificity.
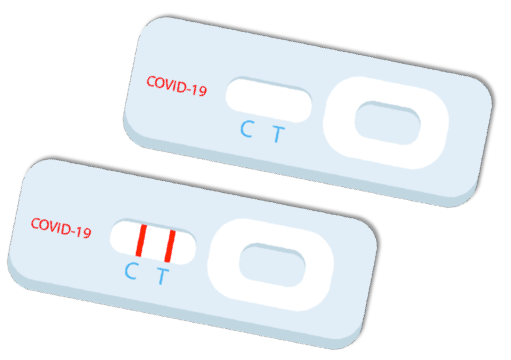
Antigen lateral flow assay cassette test

FDA EUA approved CE Marked

Human readable,
point-of-care

Results in 10 minutes with less than 12% false-negatives and 0% false-positives.

Nasopharyngeal
swab

Immediate volume available, despite 26-million-unit backlog
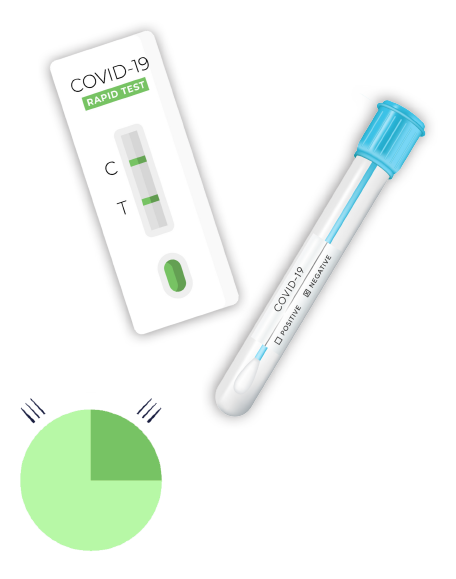
CE Marked Antigen POC Solution
Patients receive a quick and reliable result, providing the ultimate point-of-care.
Rapid results in 10 – 15 minutes with 96% sensitivity and specificity.
Antigen lateral flow assay cassette test with nasopharyngeal swab.
Test kits manufactured within the U.S. and CE Marking.
Upcoming - Rapid Digital Saliva Test
A cloud connected, highly sensitive and objective decentralized saliva antigen point-of-care test with secondary deployment for home use.
Detects trace amounts of antigen by digitally capturing fluorescent signals in the UV spectrum. The solution uses fluorescent nanoparticles which are 10x more sensitive than reflective.
Will allow for on-site testing for quick decision making with results ready in 17 minutes.
Proprietary miniaturization of digital testing can be applied to other rapid diagnostic tests.
CE Marking estimated March 1, 2021.
Not yet authorized by the FDA; undergoing FDA EUA process and subject to such regulatory approval.
In-country manufacturing and strategic alliance potential.

Questions?
Contact us today to learn more about our leading technologies!
